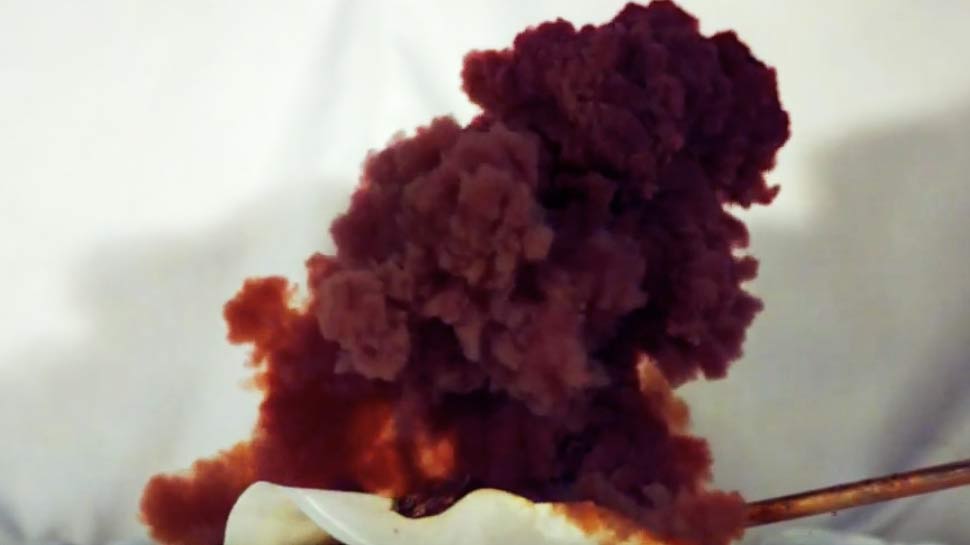
If only chemistry were this interesting back in high school, we’d have stuck with the subject for a little longer. This video by scientists at the Royal Institution shows nitrogen triiodide, a highly unstable chemical so reactive that the slightest touch can set it off, even from a stray radioactive alpha particle. A feather, a mosquito, or a gust of air can also make it burst. And when it does explodes, it goes all the way, travelling at over 7,000 miles per hour!
Nitrogen triiodide (NI3) is a black powder, produced when iodine crystals are added to a solution of concentrated ammonia in water. While the substance is wet, the water keeps it stable, but as the substance dries, it becomes wondrously unstable. The explosion results in a stunning purple plume of iodine gas and colorless nitrogen gas, the building blocks of our original molecule. “The triiodide molecules are falling to pieces and recombining into a different arrangement,” says RI. “And it’s this recombination that releases all of the energy.” The atoms are basically dancing their way through one of those painful “snowball” songs from a middle-school soirée. As energy is released in the reaction, more and more NI3 molecules follow suit, breaking apart and recombining. The chain reaction races through the substance so fast, your brain can only process it as a single, mighty blast.
Nitrogen triiodide has a small handful of uses: it’s used to kill microorganisms in flour and on melons; to clean out semiconductors; and as a fuel oxidizer. But for us dilettantes, this is the real use of this unstable chemical: awesome explosions, then breaking down the frames in slow-mo.
Of course, there’s the statutory warning: don’t try this at home, and all that.